Chemical Science & Engineering Research
Title
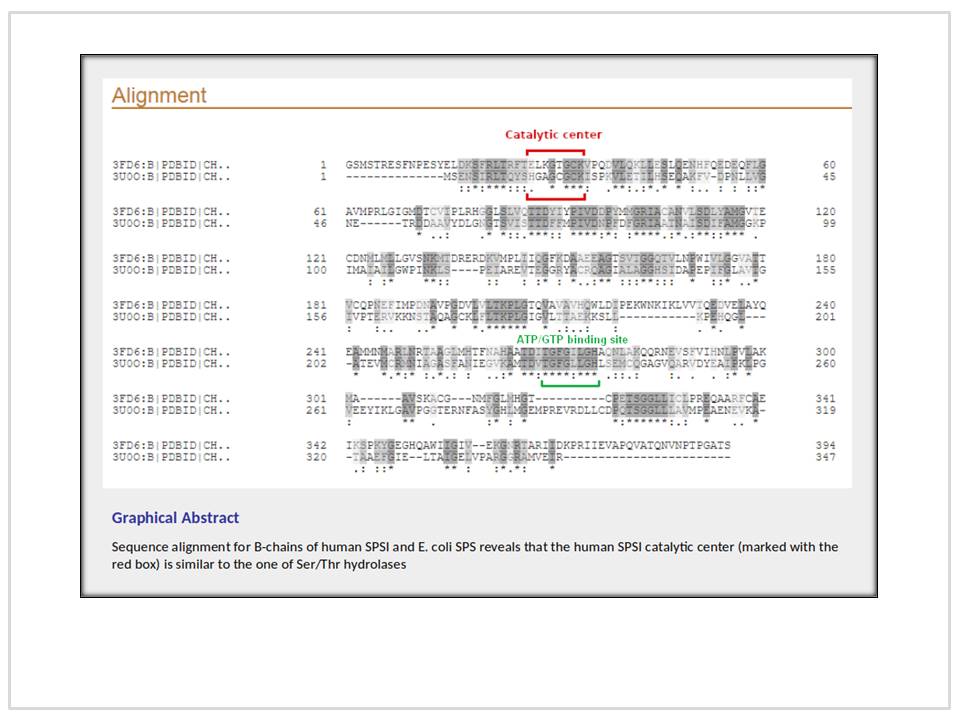
About the Role of a Human SPSI Homolog in the Living Cell (Alignments of B-Chain of Selenophosphate Synthetase (SPS) from E. coli Strain K12 (3U0O) and B-Chain of Human SPSI with Active Conformation (3FD6) and with AMP-CP as a Ligand (3FD5))
Authors
Yuliya V. Preobrazhenskaya* and Dmitry V. Preabrazhenski
Y.Kupala State University of Grodno, Grodno, Belarus 230001.
*Corresponding author E-mail address: Yuliya.Preabrazh@gmail.com (Yuliya V. Preobrazhenskaya)
Article History
Publication details: Received: 31st December 2022: Revised: 10th April 2023; Accepted: 10th April 2023; Published: 30th April 2023
Cite this article
Preobrazhenskaya Y.V.; Preabrazhenski D. V. About the Role of a Human SPSI Homolog in the Living Cell (Alignments of B-Chain of Selenophosphate Synthetase (SPS) from E. coli Strain K12 (3U0O) and B-Chain of Human SPSI with Active Conformation (3FD6) and with AMP-CP as a Ligand (3FD5)). Chem. Sci. Eng. Res., 2023, 5(12), 35-42.
Abstract
B-chains of the native selenophosphate synthetase (SPS) from strain K12 of E. coli (3U0OB) and of human SPS I (hSPSIB) was aligned with Swiss-Pdb Viewer and 24.8% identity was obtained with root mean square deviation (RMSD) of 1.48 Å. When aligned with Clustal Omega v.1.2.1, 3U0O B-chain truncated from the N-terminus till the 14th AA with B-chain of 3FD6 truncated from the N-terminus till the 13th AA, identity of 24.866% has been obtained for 93 AA. When hSPSIB and 3U0OB were aligned from the 1st AA residue with Clustal Omega v.1.2.1, 22.892% identity based on 95 AA was received using 3FD5 and 3FD6 as a reference. The aforementioned points out that the N-termini of hSPSI and the SPS from E. coli are variable. The structural alignment of the chain with the active conformation (3FD6B) and 3U0OB obtained with YASARA View shows the same 32.57% identity but lesser RMSD of 1.630 Å than the chain in inactive conformation (3FD5B), a RMSD of 1.669 Å over 218 aligned residues, under other equal parameters. It has been proposed to consider hSPSI as a Ser/Thr hydrolase.
The labelling experiments on Mn-ATP binding using the concentrations of the reagents 10-times lower then usually, 3.7 mkM WT or 3.4 mkM C17S E197D recombinant SPS and radioactive Mn-[8-14C]ATP (0.5mM), have elucidated a right shoulder in the ATP-peak observed only with WT protein in the fractions eluted from the size-exclusive HPLC column. In the labelled protein peaks under enzyme turnover conditions, Mn-[8-14C]-AMP was bound to the SPS in the amount of 10% from the total 14C loaded.
Keywords
selenophosphate synthetase I-1; alignment-2; PDB-3; sequence identity-4; RMSD-5; active center-6; Ser/Thr hydrolase-7